

Plug values in: (300 μL) / Solute Volume = 10.Choose step DFs: Need a total dilution factor of 1000.

Example: Make only 300 μL of a 1:1000 dilution, assuming the smallest volume you can pipette is 2 μL.Use the formula: Final DF = DF1 * DF2 * DF3 etc., to choose your step dilutions such that their product is the final dilution. If the dilution factor is larger than the final volume needed, or the amount of stock is too small to be pipetted, one or more intermediary dilutions may be required.
#How to do dilution series serial
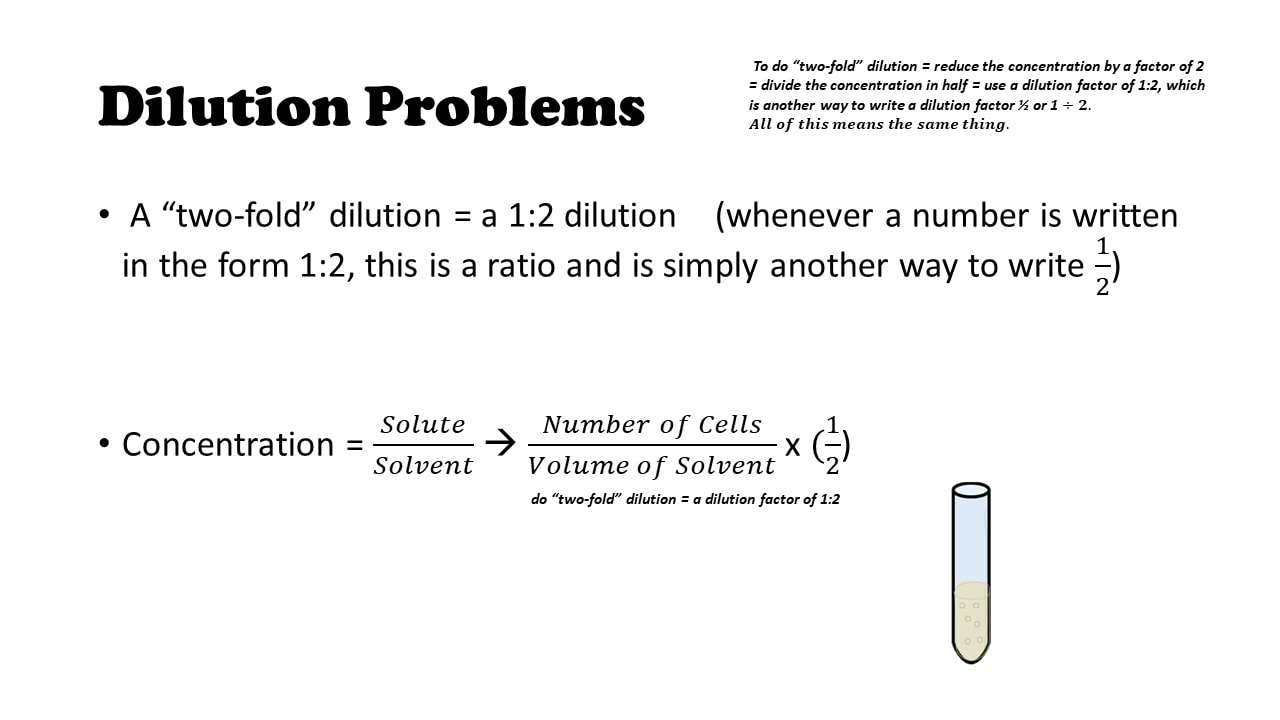
Serial dilutions are widely used in experimental sciences, including biochemistry, pharmacology, microbiology, and physics.To make a fixed amount of a dilute solution from a stock solution, you can use the formula: C 1V 1 = C 2V 2 where:

Serial dilutions are used to accurately create extremely diluted solutions, as well as solutions for experiments that require a concentration curve with an exponential or logarithmic scale. Usually the dilution factor at each step is constant, resulting in a geometric progression of the concentration in a logarithmic fashion. Subsequently, one may also ask, what does serial dilution mean?Ī serial dilution is the stepwise dilution of a substance in solution. Each dilution will reduce the concentration of bacteria by a specific amount.
#How to do dilution series series
Subsequently, question is, why is serial dilution important? A serial dilution is a series of sequential dilutions used to reduce a dense culture of cells to a more usable concentration. Serial dilutions are used to accurately create highly diluted solutions as well as solutions for experiments resulting in concentration curves with a logarithmic scale. Serial dilution is the stepwise dilution of a substance in solution. Likewise, what is serial dilution and why is it used? A serial dilution is a series of sequential dilutions used to reduce a dense culture of cells to a more usable concentration.


 0 kommentar(er)
0 kommentar(er)
